People with symptomatic HIV or AIDS at baseline gained 051kg 95 CI 036-065 p. In September 2019 the HIV monoclonal antibody ibalizumab Trogarzo received approval in the EU as a treatment for multidrug HIV resistance 18 months after approval in the US.
Some Chinese Hiv Aids Patients Give Up Free Medication To Buy Drugs From Overseas Global Times
HIV treatment drugs Pifeltro and Delstrigo doravirine lamivudine and tenofovir disoproxil fumarate were both approved in 2018 for the treatment of HIV-1 infection in.
/https://www.thestar.com/content/dam/thestar/edmonton/2019/08/30/breakthrough-hiv-medication-now-fully-covered-for-albertans/biktarvy_new_bottle_and_pill.jpg)
New hiv meds. This is a complete one-pill once-daily drug regimen. While several new antiretroviral drugs have been added to the treatment arsenal since 2010 older ones like Crixivan indinavir Invirase saquinavir Rescriptor delavirdine Videx didanosine Viracept nelfinavir and Zerit stavudine have been discontinued and are no longer in use. It can be taken with or without food.
11 Ibalizumab-uiyk is the first novel monocolonal antibody for HIV-1. Food and Drug Administration approved Trogarzo ibalizumab-uiyk a new type of antiretroviral medication for adult patients living with HIV who have tried multiple HIV medications. 60 rows Last Reviewed.
The current HIV treatment guidelines include new ARV options with better tolerability higher efficacy and lower rates of treatment discontinuation when compared with previous recommended medicines. But daily antiretroviral drugs for HIV are expensive tooand the price of those medications has risen significantly since 2012 according to a study published last year in JAMA Internal Medicine. In March 2018 the FDA approved ibalizumab-uiyk under an orphan drug designation to treat HIV-1 infection in treatment-experienced adult patients with documented multi-antiretroviral class resistance and whose regimens have failed.
People who injected drugs gained more weight than those who did not 141kg 95 CI 097-185 p. On August 30 drug manufacturer Merck announced that the FDA gave its stamp of approval to two new antiretroviral medications Delstrigo and Pifeltro. HIV works by inserting itself into the human genome and tricking the cell.
ART is recommended for everyone with HIV and people with HIV should start ART as soon as possible. A comprehensive checklist for all steps in the New Product Introduction Framework has also been developed to guide Ministries of Health and partners when introducing new. 2 3 See Table 1.
Food and Drug Administration approved Rukobia fostemsavir a new type of antiretroviral medication for adults living with HIV who have tried multiple HIV medications and whose HIV infection cannot be successfully treated with other therapies because of resistance intolerance or safety considerations. The HIV New Product Introduction Guide accompanies the toolkit and provides a guide for the user through the key steps of the New Product Introduction Framework including key activities considerations and supporting tools and resources available on the toolkit. On July 2 2020 the US.
Cabenuva the first-ever long-acting injectable HIV treatment has been approved for use by the FDA. These guidelines provide new and updated recommendations on the use of point-of-care testing in children under 18 months of age and point-of-care tests to monitor treatment in people living with HIV. And the gp-120 attachment inhibitor fostemsavir was also submitted to the FDA in December 2019 and to the EMA in January 2020 based on 96-week results from the BRIGHTE study.
People living with HIV can now opt for this treatment in place of taking a pill every day of the. The Food and Drug Administration FDA recently announced the approval of Dovato the first complete two-drug HIV treatment regimen for people who previously have not been on antiretroviral. Adults with no prior antiretroviral treatment history or.
People on ART take a combination of. In 2019 WHO recommended the use of dolutegravir-based or low-dose efavirenz for first-line therapy. Treatment with HIV medicines is called antiretroviral therapy ART.
And timing of antiretroviral therapy ART among people living with HIV who are being treated for tuberculosisNew recommendations launched today outline. New injectable HIV treatment will help those who struggle with daily pills. The treatment monitoring algorithm.
Cabenuva cabotegravir rilpivirine NA. This is a long-acting injectable regimen administered every four weeks. Each tablet contains 50 mg bictegravir 25 mg tenofovir alafenamide and 200 mg emtricitabine.
ViiV Healthcares Dovato was originally approved in April 2019 as a complete HIV regimen for those with no history of antiretroviral treatment and with no known resistance to the two components of the daily pill dolutegravir and lamivudine. 1 2020 Dolutegravir the current first-line treatment for HIV may not be as effective as hoped in sub-Saharan Africa suggests new research published on.
 New Affordable Hiv Treatment Vuk Uzenzele
New Affordable Hiv Treatment Vuk Uzenzele
Bay Area Reporter Fda Approves New Hiv Meds
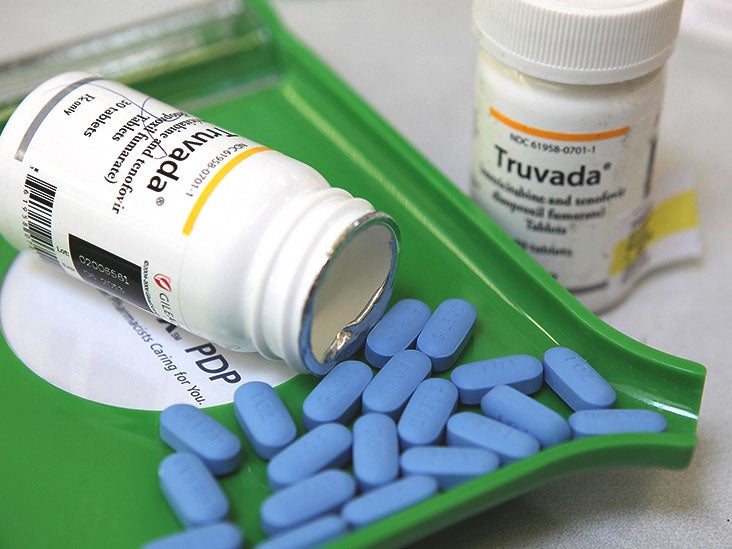 New Two Drug Regimen Can Treat Hiv
New Two Drug Regimen Can Treat Hiv
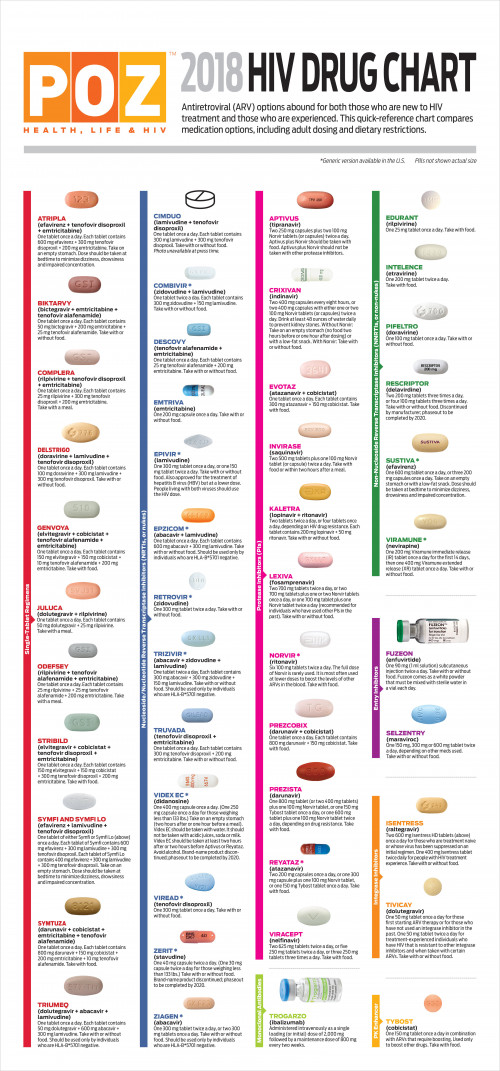 Chart Of Hiv Medications Canada
Chart Of Hiv Medications Canada
 Here S What You Need To Know About Hiv Drug Resistance San Francisco Aids Foundation
Here S What You Need To Know About Hiv Drug Resistance San Francisco Aids Foundation
 Start And Continue Hiv Meds As Soon As You Can San Francisco Aids Foundation
Start And Continue Hiv Meds As Soon As You Can San Francisco Aids Foundation
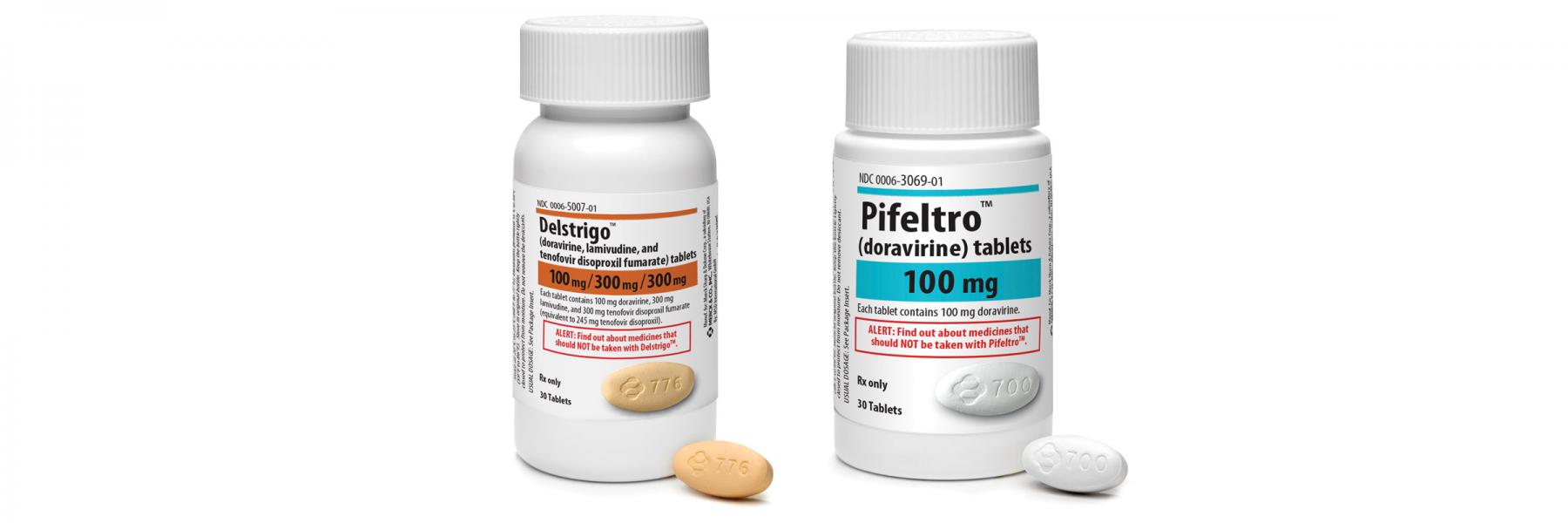 Briefly Two New Hiv Meds Approved Delstrigo And Pifeltro Positively Aware
Briefly Two New Hiv Meds Approved Delstrigo And Pifeltro Positively Aware
 Janssen Wins Fda Nod For Symtuza But The Once Daily Hiv Drug Must Now Find Its Place In The Market Fiercepharma
Janssen Wins Fda Nod For Symtuza But The Once Daily Hiv Drug Must Now Find Its Place In The Market Fiercepharma
/https://www.thestar.com/content/dam/thestar/edmonton/2019/08/30/breakthrough-hiv-medication-now-fully-covered-for-albertans/biktarvy_new_bottle_and_pill.jpg) Breakthrough Hiv Medication Now Fully Covered For Albertans The Star
Breakthrough Hiv Medication Now Fully Covered For Albertans The Star
 Berry Flavored H I V Medication Is Ready For Babies The New York Times
Berry Flavored H I V Medication Is Ready For Babies The New York Times
 Will The New Prep Pill For Hiv Prevention Fuel Progress Or Profits Stat
Will The New Prep Pill For Hiv Prevention Fuel Progress Or Profits Stat



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.