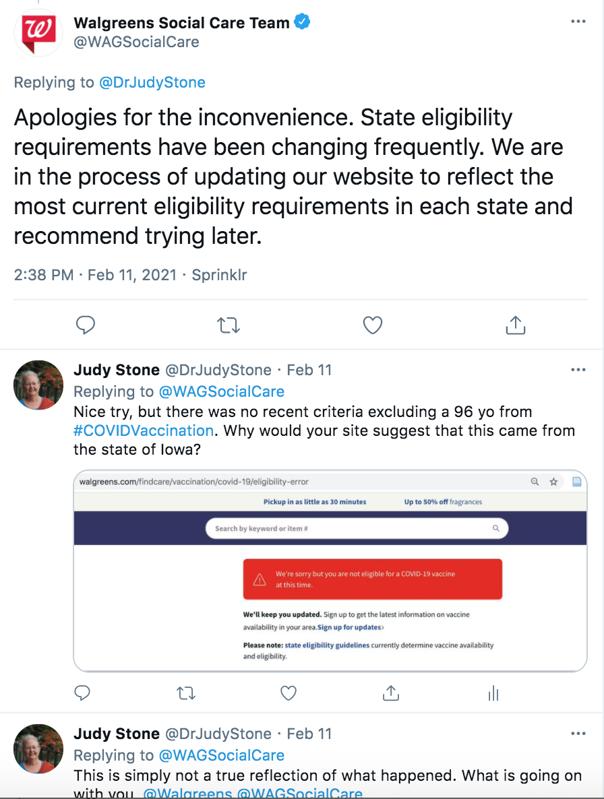
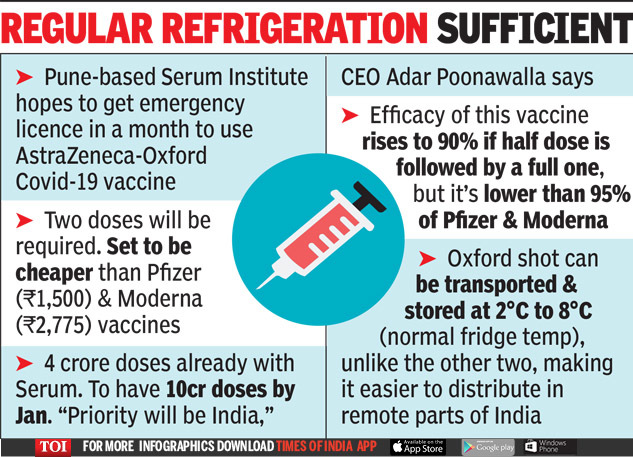
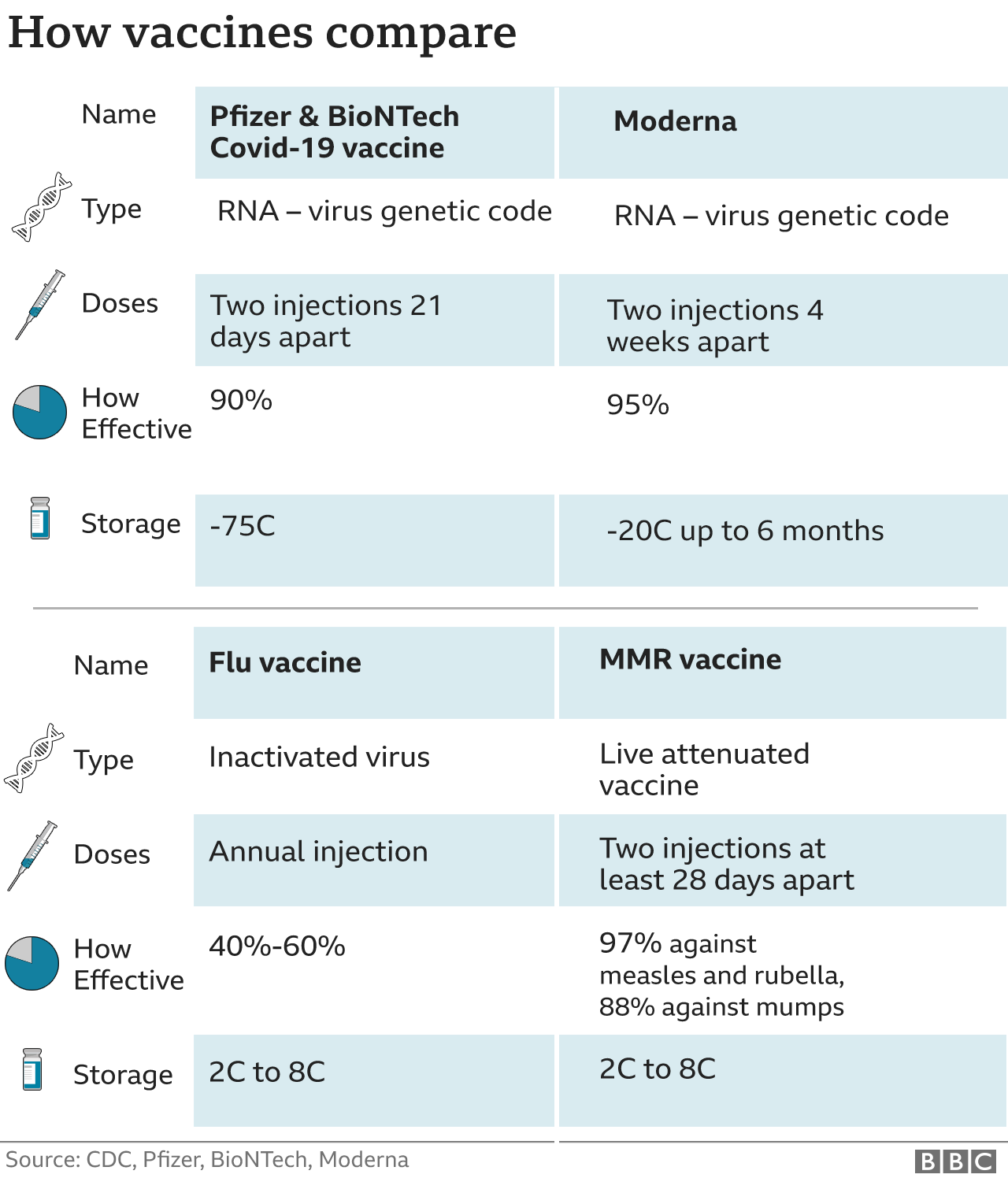
Preregister for state mass vaccination sites here. The State of Marylands plan for this historic undertaking will immediately make the vaccine available to Marylanders at highest risk of developing complications from COVID-19 as well as our.
 Covid 19 Vaccine Resources Maryland Developmental Disabilities Council
Covid 19 Vaccine Resources Maryland Developmental Disabilities Council
The governors office released the details of Marylands two-phase strategy on Tuesday.

Plan your vaccine maryland. Centers for Disease Control and Prevention. Plan your vaccine Maryland Virginia and DC. Pre-register online to schedule an appointment at a mass vaccination site at massvaxmarylandgov or by calling states COVID-19 vaccination support center at 855-634-6829.
Safe and Phased Plan for Vaccination Distribution Marylands draft COVID-19 vaccination plan focuses on two major phases of vaccine availability and distribution. If I live in one state can I travel to get a vaccine in another state. Maryland will move to Phase 1B of the states COVID-19 vaccination plan beginning Monday Gov.
Most of these jurisdictions do not have a plan said Bauman. Call the State Vaccine Support Center at 1-855-634-6829. Despite dentists being included in phase one of the vaccine distribution in Maryland according to the Maryland State Dental Association only three counties have started vaccinating dentists.
Maryland Governors Office Maryland vaccination phases. ALL MARYLANDERS 16 ARE ELIGIBLE TO RECEIVE THE VACCINE. FAQs about the Maryland COVID-19 Vaccination Plan and COVID-19 Vaccination Trending Questions and Answers COVID-19 Vaccination Plan COVID-19 Vaccines Registering for a COVID-19 Vaccine.
Visit COVIDLink to learn more about the COVID-19 vaccination phases and eligibility in Maryland. All the information you need by county. Inclement weather may impact appointments and scheduling.
Please contact the provider to confirm availability. The State of Marylands plan for this historic undertaking will immediately make the vaccine available to Marylanders at highest risk of developing. There are many ways to schedule a vaccine in Maryland.
As the federal government gears up for a potential COVID-19 vaccination campaign Maryland has submitted its distribution plan to the US. The Plan Your Vaccine page will be updated as new information is released from state health departments. Hard copies can be downloaded for printing and distribution.
If you are a healthcare worker who cant access the vaccine through your employer call the local health department where you work or contact the VDH COVID-19 Call Center at 877-275-8343 for. We are 3-4 weeks into giving of these vaccines right now. COVID-19 Vaccine All Marylanders 16 and older are eligible for vaccination.
MARYLAND USA Making a COVID-19 vaccination appointment in Maryland is a three-step process. Vaccination Phases Maryland is currently in Phase 3. Phase 1 will focus on priority groups to receive vaccination and Phase 2 will have wide-scale vaccine availability for the general population.
The center is open 7 days a week from 7 am. Most are still collecting. Here they are listed below.
States were due to submit draft plans to the CDC by last week. Though the hype for an immunization is swirling the governor. Opt-Out Form - Use this form if you do not wish to share your or your childrens vaccination records with providers in ImmuNet.
1 determining which phase you fall into 2 pre-registering or filling out an interest form if. Larry Hogan announced Tuesday. Pre-register for an appointment at a mass vaccination site or find a pharmacy hospital or local health department near you to make an appointment.
A site is now open at Montgomery Colleges Germantown campus along with a federally-backed community vaccine site at the Greenbelt Metro station. Mass vaccination sites. Vaccination Records Request - Get your or your childrens vaccination records securely by registering at MyIRSee this page for more information about MyIR.
The Maryland Department of Health has also launched a support center focused on vaccine outreach for the states vulnerable populations. ANNAPOLIS MD Maryland is preparing to roll out a coronavirus vaccine as soon one is authorized Gov.